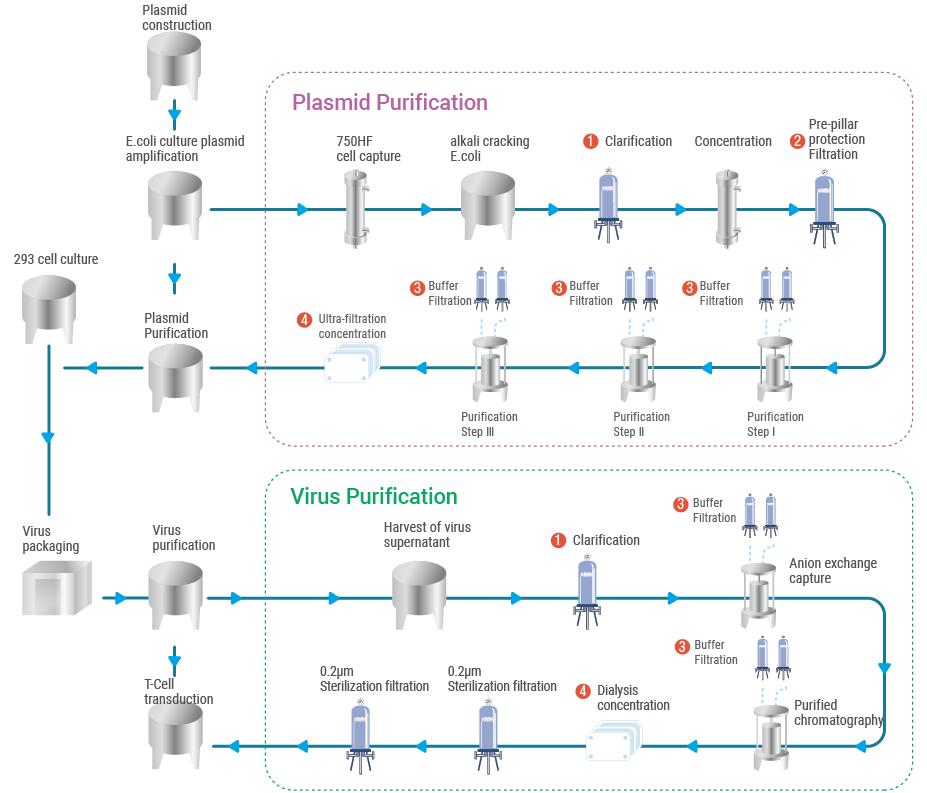
Gene therapy refers to the introduction of exogenous normal genes into target cells to correct or compensate for diseases caused by defective and abnormal genes, in order to achieve therapeutic purposes. Theoretically, gene therapy can cure most diseases, but because the occurrence of diseases often involves multiple genes, the interaction between corresponding proteins forms a huge regulatory network, and it is difficult to regulate only one or a few genes to achieve the purpose of treatment.
In addition, the safety of gene therapy has always been of great concern to people. The development of gene therapy technology had its ups and downs until 2012, when the Dutch company UniQure's Glybera was approved and marketed in the European Union for the treatment of severe muscle diseases caused by lipoprotein lipase deficiency. In the same year, Jennifer Doudna and Chinese-American scientist Feng Zhang invented the CRISPR/CAS9 gene editing technology, which was a revolutionary event in the field of gene therapy. So far, the bottleneck of gene therapy technology has been broken through, and the effectiveness and safety of gene therapy has been improved, and the industry has ushered in another wave of development. Gene therapy can be divided into in vivo and "in vitro" treatment from the process. The most successful aspect of "in vitro" treatment is cell therapy, which refers to the modification, cultivation and expansion of somatic cells in vitro, and then transfused back to the patient. In particular, the maturing of Car-T technology is expected to make cell therapy the next pinnacle of the field after antibodies. The market value is expected to exceed $34 billion by 2025. However, there are still many bottlenecks that need to be overcome in cell therapy. Since only autologous immune cell therapy can be realized at present, the production process can only be a large-scale, parallel, customized production, which is much more complex. At the same time, it is a "living" drug that requires a sustainable and effective cure process, and the "Chain of custody" associated with the patient throughout the transport, preparation and treatment process further increases costs.