PES Membrane
 Cobetter's PES membranes are widely used hydrophilic membranes in the pharmaceutical industry, with high resistance to solutions ranging from pH 1-14, organic solvents. It can resist the temperatures up to 150°C.
Cobetter's PES membranes are widely used hydrophilic membranes in the pharmaceutical industry, with high resistance to solutions ranging from pH 1-14, organic solvents. It can resist the temperatures up to 150°C.
And it is usually used for liquid sterilization and de-mycoplasma filtration.
Cobetter's polyethersulfone (PES) membranes use a variety of membrane manufacturing processes to produce PES membranes with great performance.
The symmetrical structured membrane has stable sterilization capacity; the asymmetrical structured membrane greatly improves the contaminant holding capacity and prolongs the service life. Its service life is 3-5 times longer than other similar PES products, and its double-layer hydrophilic structure gives it great filtration performance and reliable bacterial retention to meet the filtration requirements of pharmaceutical processes.
Cobetter also has several series of polyethersulfone products to meet the different filtration needs of a large number of customers. For example, liquid bacterial cartridges with low diffusion flow, sterilization grade cartridges with triple lifetime compared to ordinary polyethersulfone, low protein adsorption and hydrophilic sterilization cartridges, and low protein retention cartridges, high-flow rate hydrophilic sterilization cartridges, etc.
In addition, Cobetter has also developed a TFF membrane made of polyethersulfone, which offers high throughput, low extractables, and high efficiency. It has the advantages of great chemical compatibility, low non-specific protein adsorption, and high product recovery rate.
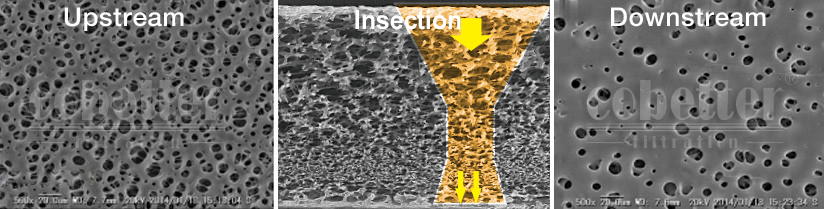
- Meet 107cfu/ cm² Brevundimonas diminuta (ATCC*19146) test according to ASTM F838 test method.
- Each filter cartridge undergoes integrity testing before delivery
- Meets the definition of "non-fiber releasing" filter as defined in FDA 21 CFR 210.3(b)(6). standard
- In-line steam sterilization at 135 °C for 30 min, up to 100 cycles.
- Autoclavable at 130 °C for 30 min, cumulative 200 cycles
- Double-layer design ensures reliable bacterial retention with good longevity and low cost.
- Highly consistent, stable batch-to-batch variance
- Broad chemical compatibility, pH 1-14, effective for a wide range of pharmaceutical filtration processes.
Removal Ratings (μm)
0.1 / 0.22 / 0.45 / 0.65 / 0.8 / 1.2 / …
0.22+0.1 / 0.22+0.22 / 0.45+0.22 / 0.45+0.45 / 0.65+0.22 / 0.8+0.45 / 1.0+0.6 / …
Typical Application
- Sterilization and filtration of biological products (such as vaccines, monoclonal antibodies, blood products, recombinant proteins, etc.)
- Sterilization and filtration of buffer and culture medium
- Filtration of lyophilized preparations
- Sterilization and filtration of antibiotic solutions
- Eye drops for sterilization and filtration
- Sterilization and filtration of LVP and small SVP.
- Sterilization and filtration of physiological saline and other solvents
- Sterilization and filtration of large quantities of pharmaceutical, cleaning and disinfection solutions





































 Cobetter's PES membranes are widely used hydrophilic membranes in the pharmaceutical industry, with high resistance to solutions ranging from pH 1-14, organic solvents. It can resist the temperatures up to 150°C.
Cobetter's PES membranes are widely used hydrophilic membranes in the pharmaceutical industry, with high resistance to solutions ranging from pH 1-14, organic solvents. It can resist the temperatures up to 150°C. 







