Current Situation of Corona Virus(Covid-19)
Since the Corona Virus was discovered and outbreak from January 2020 till 24th of March 2020, a total accumulated cases of 81,737 had been diagnosed in China and the current confirmed cases is 5189 cases. In the mean time, the abroad nations were facing the epidemic’s rise, countries like Japan, Korea, Italy, United States of America had a large-scale numbers of outbreaks and many of confirmed cases had been discovered. As of today, There is about 298,331 of accumulated cases and 257,557 of current confirmed cases on abroad.

Succeed Developed of Corona Virus Test Kits
As during the early stage of epidemic’s outbreak, Chinese IVD companies took a quick steps into the R&D of virus test kits and these test kits were successfully developed within couples of months. According to the statements that issued by the State Drug Administration of China on 16th March, as of today, there were 11 test kits and 8 antibody kits been approved. As for now, there are more than 64 companies had self-declared that their virus test kits had obtain the EU admission qualification or CE certificate. Basically there are two types of virus test kits, for nucleic acid detector kit, this kit implied a multiple PCR-Fluorescent probe detection method combined with RT-PCR technology which may sampling the suspected cases of Corona Virus infection and its related genes that will be tested.
For antibody kit, the lateral flow of immunochromatography will used. IgM and IgG antibodies could be detected indirectly.
The launch of these two tester kits had provide a significant aids for government, especially in terms of diagnoses and detection of Corona Virus.
Lateral Flow Immunochromatograph Assays
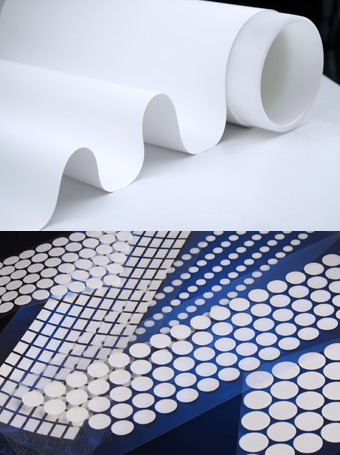
Compare to nucleic acid detection platform, lateral flow immunochromatograph assays has a faster detection speed(5-30 mins), low instrument dependence, friendly user, low cost, could be tested separately, this kit had been widely accepted and used by clinical as well as third-party institutions. Depending on the type of marker, it could be colloidal gold immunochromatography, fluorescent quantitative immunochromatography. Capillary action of sample solution that added to one end of membrane in immunochromatography(based on the cross flow of tomography) move to the other end, analytes and receptors fixed to a certain area on the membrane during the movement( antigen or antibodies) were solidified, irrelevant substances are separated across the area, the test results are then judged by the color development of the marker. Lateral flow device as shown below, which contains of sample pad, hemofiltration membrane, bonding pad, chromatography membrane and absorption pad. During the corona virus test that takes whole blood sample, the material of the hemofiltration membrane is very important. This membrane will filters whole blood samples that pass through, and retains red blood shell and other components that are not needed. Which plasma could be isolated for the next subsequent reactions. The process of filtration and separation will have a high demand on the ability of hemofiltration membranes to separate red blood cells and plasma. The key points were: firstly, whether the erythrocyte filtration rate is high enough; Secondly, whether plasma recovery rate could be kept high and stable. Lastly, whether the diffusion rate is fast enough.

Cobetter's plasma seperation membrane, applied to Lateral Flow Immunochromatography Assays
Cobetter as a professional membrane's supplier in the field of diagnostics, fully understanding the high technical requirement and performance stability of membranes for various application in the diagnostic field. A group of specialized diagnostic application engineers & Research and Developments engineers had been formed and taken place. In-depth understanding and learning various applications in the field of diagnostics, and finally apply these to the membrane development and production quality control, to ensure reliable quality, high performance and stable membrane material for our customers.


Since the outbreaks of Corona Virus, more and more IVD companies involved into the R&D and production of virus test kits, various product had been through the approval of China’s State Drug of Administration and even exported to overseas, so that could be used in the front-line of Global outbreak prevention. Hemofiltration membrane as the core material of Corona Virus detector kit, currently faced a great demand for supply. Imported hemofiltration membrane are slower in delivery time, higher price and poor services. Cobetter provides customers with the great quality, shorter delivery time and a outstanding membrane. Welcome for any inquiries, contact e-mail: sales@cobetterfilter.com.